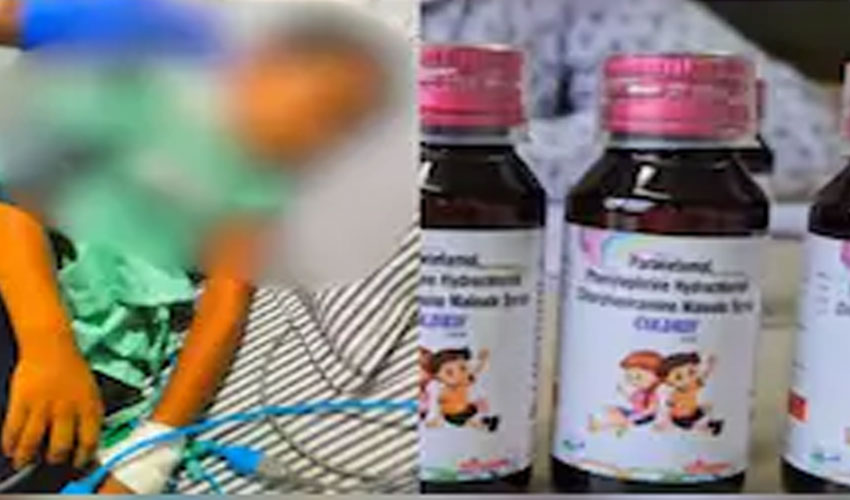
Authorities have arrested the owner of Sresan Pharmaceutical Manufacturer, whose cough syrup has been linked to the deaths of at least 17 children in Madhya Pradesh over the past month.
All of the victims—children under the age of five—reportedly consumed ‘Coldrif’, a cough syrup later found to contain diethylene glycol, a toxic chemical nearly 500 times above the permissible limit.
Police confirmed that S. Ranganathan, the owner of the Tamil Nadu-based drug company, was arrested on Wednesday in Chennai. According to Chhindhwara Superintendent of Police Ajay Pandey, Ranganathan will be produced in court and then transferred to Madhya Pradesh for further investigation.
The syrup in question, Coldrif, has now been banned in several parts of India, following laboratory tests that revealed the presence of the deadly chemical compound.
Regulatory failures under scrutiny
Indian law mandates pharmaceutical companies to test every batch of raw materials and finished products. However, this case has raised serious concerns about lapses in quality checks and enforcement.
Since 2023, India has also required export-bound syrups to undergo additional testing at government-approved laboratories—after similar poisoning incidents in Gambia, Uzbekistan, and Cameroon resulted in the deaths of more than 10 children.
The World Health Organization (WHO) has called this latest case evidence of a “regulatory gap” in India’s domestic medicine screening. The agency also warned that unofficial exports of contaminated syrups might have occurred.
Indian health authorities have also warned against two other locally sold syrups—Respifresh and RELIFE—manufactured by Gujarat-based Shape Pharma and Rednex Pharmaceuticals. Tests showed these products contained the same toxic chemical, diethylene glycol.
Both companies have not responded to media inquiries so far.
India’s reputation at stake
India is the world’s third-largest drug producer by volume, trailing only the United States and China. The country is known as the “pharmacy of the world”, supplying about 40% of generic medicines used in the U.S. and more than 90% of essential drugs in several African nations.
This latest scandal has reignited debates over drug safety oversight, especially for products made for domestic consumption.